electrolysers
Hydrogen production via PEM electrolysers

In first place!
Hydrogen is the first element in the periodic table and offers countless technological possibilities. hähn hydrogen offers you hydrogen production using PEM electrolysers.
The systems are available as compact and containerized systems. If required, hähn can also supply the corresponding peripherals.
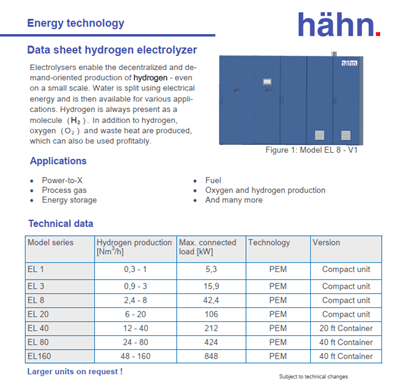
The electrolysers enable decentralized and demand-adapted production of hydrogen - even on a small scale.
The performance classes offered can be found in the data sheet.
Your benefits
Modular systems with various options - expandable at any time
Support with questions about funding opportunities
Robust industrial design
No hazardous chemicals, therefore no WHG
No compression, no compressor required
Plug and use systems
Suitable peripheral systems are also available from us
If required, we can also provide you with coordinated systems for further processing of your hydrogen. Find out more about the energetic use in our fuel cell systems and the material use in our power-to-X plants.
The risk of fire or explosion when handling hydrogen is comparable to that of natural gas or other conventional energy sources.
Data sheet haehn hydrogen electrolyzers (pdf)
Knowledge to go
- General information
-
Under normal conditions, hydrogen exists as an H2 molecule, a colorless and odorless gas. It is the lightest element in the periodic table of elements and is therefore in first place.
Hydrogen is the most common element in the universe and a component of water and all living things.
Hydrogen as an energy carrier can make renewable energies "sustainable". Green hydrogen plays a decisive role in the decarbonization of the energy sector, industry and mobility.
The first electrolysis was carried out back in 1800.
- Technical data
-
Calorific value: 3 kWh/ Nm3 - 33.33 kW/kg
Density: 0.0899 kg/Nm3
Energy content of 1 Nm3 hydrogen corresponds to 0.34 l gasoline
1 kg hydrogen corresponds to 2.75 kg petrol or approx. 2.1 kg natural gas --> Highest energy density by mass
Hydrogen is
- non-explosive (in pure form), non-toxic, non-corrosive, non-combustible, non-flammable, non-decomposable, non-radioactive, non-odorous, non-hazardous to water, non-carcinogenic
- about 14 times lighter than air and very volatile. This leads to rapid mixing to harmless concentrations with the ambient air
- burns with oxygen only to water
- Application areas
-
Hydrogen is a versatile energy carrier and raw material supplier and offers countless technological possibilities across sectors (e.g. energy, transportation, chemicals).
Hydrogen is therefore an energy carrier and raw material that will play a decisive role in the overall economy in the coming decades.
Its tasks range from long-term energy storage and thus full utilization of PV and wind farms, direct feed-in into the natural gas grid or demand-adapted reconversion into electricity in fuel cell systems to its function as a raw material supplier in synthesis plants (Power-to-X).
- Energetic use
-
Hydrogen technology can not only be used to supply extremely energy-intensive industrial processes, e.g. in steel production. Our energy system, the chemical industry and mobility can also benefit effectively from its use as an energy source. Hydrogen can serve both as a long-term storage medium and as a producer of electrical energy.
Hydrogen can be used as an additive directly as an energy source in the conventional natural gas grid or converted back into electricity using fuel cell systems.
These are used as power generators and CHP units, emergency power systems, in the area of balancing energy, or as power pacs in microgrids or for emission-free and noise-reduced drives in shipping, rail or heavy goods transport and for work machines.
- Material use
-
In addition to its energy potential, hydrogen offers countless opportunities for material processing.
Hähn hydrogen offers a range of Power-to-X technologies (PtX) to exploit hydrogen as a raw material supplier. Hydrogen is used as a synthesis gas in chemical production plants, for example to produce fertilizers, low-CO2 synthetic fuels or basic chemicals.
The plants available from hähn hydrogen include
- Haber-Bosch synthesis: ammonia production
- Fischer-Tropsch synthesis: hydrocarbons such as petrol, kerosene, kerosenes, basic chemicals
- Methanol synthesis: methanol as energy storage, higher energy density than H2 and can be stored in liquid form
- Hazard analysis
-
Hydrogen is not self-igniting. Nevertheless, it is classified as a hazardous substance, as it can explode at a certain mixing ratio with air and e.g. a spark (oxyhydrogen reaction). The hazard potential is similar to that of natural gas.
However, hydrogen is about 14 times lighter than air and very volatile. This leads to rapid mixing to harmless concentrations with the ambient air.
It has been tried and tested in the chemical industry in particular for over 100 years with very good safety ratings.
Hydrogen is no more dangerous than a conventional energy source such as natural gas.
- Green hydrogen
-
Green hydrogen is produced by electrolyzing water in electrolysers. The electricity required for this comes from renewable sources, e.g. from energy surpluses. Due to its properties and flexibility, green hydrogen plays a decisive role in the decarbonization of the energy industry, mobility and production. It can be used to preserve renewable energies over a long period of time.
It plays a key role in the German government's energy transition, but also internationally. hähn hydrogen is also at your side when it comes to funding opportunities!
Thanks to its storage capacity and high specific energy density, large amounts of energy can be stored for months. By using renewable energies, this is the most climate-friendly method of storing energy on a large scale. In addition, energy surpluses can be used profitably, which in turn significantly improves the profitability of PV or wind farms.
Jules Verne (1828-1905) is also known to have said: "Water is the coal of the future"
- Electrolyzers
-
During electrolysis, water is split using electrical energy. The resulting hydrogen is then available for various applications. Hydrogen is always present as a molecule (H2). In addition to hydrogen, oxygen (O2) and waste heat are produced, which can also be used profitably.
The first electrolysis was carried out over 200 years ago.
- PEM-technology
-
PEM electrolysers are currently the primary technology used. It is a membrane-based process for splitting water into oxygen and hydrogen with a solid electrolyte. PEM stands for proton exchange membrane or polymer electrolyte membrane.
The so-called "stack" contains electrolysis cells that contain a catalytically coated membrane. This is where the splitting of water into oxygen, free electrons and protons takes place under electrical voltage, as well as the combination to form H2. The membranes are only permeable to protons. Together with the anode and cathode, the construct forms an electrolytic cell. These are installed in the electrolysis stacks from hähn hydrogen.
iph Hähn GmbH
Konrad-Zuse-Str. 9 | DE-53560 Vettelschoß | fon 02644-98 02 96 | info@iph-haehn.de | www.iph-haehn.de
Images on this page from top left to bottom right: iph Hähn GmbH

